Hbv Dna Test In Hindi
Gale Onefile Health And Medicine Document Applicability Of Oral Fluid And Dried Blood Spot For Hepatitis B Virus Diagnosis
Hcv Rna Pcr What To Know About Hepatitis C Testing
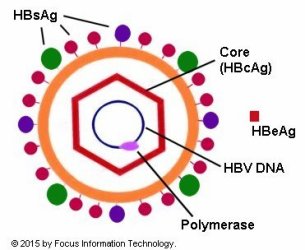
Hepatitis B In Pregnancy

Hepatitis B In Hindi ज न क य ह त ह ह प ट ट स ब By Dt Radhika Lybrate

Doc Price List In Hindi Anji Gurram Academia Edu

Serum Hepatitis B Surface Antigen Levels Correlate With High Serum Hbv Dna Levels In Patients With Chronic Hepatitis B A Cross Sectional Study Gupta E Kumar A Choudhary A Kumar M Sarin S K
Alcohol represents the most common cause of HCC in Italy and the US, accounting for 32% of all HCC cases in Italy and 35% in the US.
Hbv dna test in hindi. The test is most often used to monitor the efficacy of antiviral therapy in individuals with chronic HBV infection. Below the threshold, the viral load is considered “undetectable” – something everyone with chronic hepatitis B wants to hear. A simple blood test can let a person know if the hepatitis B virus is in their blood or if they have successfully gotten rid of the virus.
You will get a discount of up to 46% over the market price on HBV DNA Quantitative PCR price in Ghaziabad when booking through us. In one investigation using the Amplicor HBV monitor test (reported sensitivity of 400 copies/mL;. The Health Department currently requires laboratories to report only positive HBV DNA results, in addition to other positive HBV test results.
हेपेटाइटिस बी डीएनए टेस्टिंग (Hepatitis B DNA testing) – इस डीएनए के उच्च स्तर का मतलब होता है कि वायरस आपके शरीर में गुणात्मक रूप से बढ़ रहा है और आप. Anti-H & HBV-DNA detection in blood donors negative for hepatitis B virus surface antigen in reducing risk of transfusion associated HBV infection Indian J Med Res. This test helps to distinguish active from an inactive infection in Hepatitis B chronic infected individuals.
Information for health care providers on interpreting pre-vaccination testing for hepatitis B, from the VA National Viral Hepatitis website. This assay detects all hepatitis B virus (HBV) genotypes from A to H. HEPATITIS B VIRAL (HBV DNA) QUANTITATIVE, REAL TIME PCR Result/s to follow:.
– INR – Total bilirubin – ALT (weekly) – Supportive care and hydration. Freeze (preferred) or refrigerate. You will get a discount of up to 10% over the market price on HBV DNA Viral Load Quantitative Test price in Mumbai when booking through us.
HBV viral loads detected at less than IU/mL are not quantitated. Limitations The quantitative test has a range of 10 IU/mL to 1,000,000,000 IU/mL. There are 3 common tests that make up this blood panel.
HBV DNA testing checks for genetic material (DNA) from the hepatitis B virus. After 4 years off treatment, in the evaluation cohort, 30.8% of patients had a clinical relapse, 54.7% had virologic relapse (HBV DNA >00 IU/mL in 2 tests), and 16.8% had reappearance of HBeAg in 2 tests (reversion). This blood sample can be taken in the doctor’s office.
The method detecting HBV DNA in serum by HBV DNA AlkPhos Direc probe is sensitive and specific. In addition, there was a significant relationship between HBV DNA values in the blood and sweat of the wrestlers (r = 0.52, p<0.01). Test for HBV DNA may not be sensible enough or collection of the sample is incorrect;.
Although the diagnosis of acute and chronic HBV infection is usually made by serologic methods, detection and. The detectable HBV DNA was significantly associated with a higher risk of HCC development compared with the continuously undetectable HBV DNA (HR 2.442;. There is a simple hepatitis B blood test that your doctor or health clinic can order called the “hepatitis B blood panel”.
17 In this study, when patients received a. Specimen 3 mL (2 mL min. It also helps to monitor and evaluate the treatment.
A correlation coefficient of HBV DNA quantitative results between fluorescent quantitative HBV DNA PCR (QPCR) method and dot blot hybridization assay by AlkPhos Direc probe was 0.98 (P<0.01). This assay targets the highly conserved pre-Core/Core region of the HBV genome. IgM anti-H – a positive blood test result indicates a person has a new acute hepatitis B infection.IgM anti-H is generally detectable at the time.
• Has a wide linear range to accurately quantitate HBV viralload in patients with chronic HBV infection undergoingantiviral drug therapy. Stable frozen for 12 weeks. This video explains about Hbeag test, hbeag test means, hbeag test means in hindi, hbeag negative means in hindi, hbeag normal ranges, hbeag test in hindi, hbeag, hepatitis, hepatitis b, hbsag.
Further testing for HBV-DNA would be appropriate to follow up the donor for HBV infection. The lab nearest to you for HBV DNA Viral Load Quantitative Test in Mumbai is just 1.45 kms away. For your HBV DNA Quantitative PCR in Ghaziabad more than 11 certified labs are available.
Hepatitis B DNA Viral Load PCR Blood Test measures the amount of Hepatitis B viral load or Hepatitis B virus DNA (HBV DNA) levels in the blood of chronically infected patients. • Targets highly conserved regions within 2 genes for addedprotection against HBV mutations. Assay is the FDA approved Roche AmpliPrep/COBAS®TaqMan® HBV Test.
For your HBV DNA Viral Load Quantitative Test in Mumbai more than 11 certified labs are available. Quantitative nucleic acid test for use on the cobas® 6800/00 systems. Hepatitis B Surface Antigen positive.
₹ 16,900.00 ₹ 13,000.00 Book Test your DNA allows you and your physician to make better choices for your health Your DNA is composed of a very long string of molecules — 6.4 billion A’s, T’s, C’s and G’s. Common tests used by doctors to monitor your hepatitis B include the hepatitis B blood panel, liver function tests (ALT), hepatitis B e-Antigen (HBeAg), hepatitis B e-Antibody (HBeAb), hepatitis B DNA quantification (viral load),and an imaging study of the liver (ultrasound, FibroScan Transient Elastography or CT scan) biopsy before starting. This test is intended for use as an aid in management of HBV infected patients and is not intended for use in the initial diagnosis or confirmation of HBV infection.
Please submit a separate frozen specimen for each test requested. Findings of chronic HBV (HBsAg-. HBeAg (hepatitis B e-antigen) is generally detectable in patients with a new acute infection;.
The Aptima HBV Quant assay is licensed for the quantitation ofHBV DNA on the fully automated Panther system.This assay:. The FDA issued a draft guidance recommending the use of FDA-licensed hepatitis B virus infection nucleic acid tests for donors of human cells, tissues and cellular and tissue-based products in an. Chronic Hepatitis B Chronic suspected if anti.
For your HBV DNA Quantitative PCR in Delhi more than 12 certified labs are available. This test is intended for use as an aid in management of HCV infected patients and is not intended for use in the initial diagnosis or confirmation of HCV infection. Amid the simmering debate over the language politics in Tamil Nadu, a retired doctor from Ariyalur district's Gangaikondacholapuram has alleged that he and an accompanying engineer were treated disrespectfully and denied a loan by the manager of an Indian Overseas Bank branch, for not knowing the Hindi language.
The HBV DNA tests measure how much genetic material is present. Santosh medical awareness campaign 3,2 views 3:28. HBsAg (hepatitis B surface antigen) is the first serologic marker to appear in a new acute infection, which can be detected as early as 1 week and as late as 9 weeks, with an average of one month.
Reduction of window-period HBV transmissions through detection of HBV DNA-positive units by minipool nucleic acid testing (MP NAT) would be expected to decrease this risk. The sensitivity of HBV DNA tests may vary with each lab so it’s a good idea to use the same lab for your test. Hepatitis B Virus (HBV), Quantitative, DNA Real-time PCR, (Graphical) TEST:.
This test quantitates HBV DNA in serum or plasma with reflex to HBV genotype if ≥500 IU/mL is detected. 16 Hepatitis B virus precure and core region mutations might be associated with HCC tumorigenesis. A significantly lower proportion of double negative patients had a clinical re ….
Apply for and manage the VA benefits and services you’ve earned as a Veteran, Servicemember, or family member—like health care, disability, education, and more. 10 High HBV DNA level is the main risk factor for hepatocarcinogenesis. Two FDA-approved ( in vitro diagnostic IVD) hepatitis B virus (HBV) viral load assays, the manual Cobas TaqMan HBV Test for use with the High Pure System (HP) and the automated Cobas AmpliPrep/Cobas TaqMan HBV Test v2.0 (CAP/CTM), were compared to a modified (not FDA-approved) version of the HP assay by automating the DNA extraction using the Total Nucleic Acid Isolation (TNAI) kit on the.
It may be used to determine the following, To check whether acute symptoms are due to HBV infection For diagnosing chronic HBV hepatitis To monitor chronic condition of hepatitis B infection and effectiveness of treatment To identify previous exposure to hepatitis B infection To determine if a person is carrier of the infection during. Roche Diagnostics, Raritan, NJ), HBV DNA was detectable in only eight of 15 patients positive for hepatitis B DNA on nested PCR. Print Share Include LOINC® in print.
On the other hand, according to real time PCR for serum HBV DNA detection in this study, 9 (13%) of the wrestlers had OC‐HBV infection. The Department is proposing that the Board amend Health Code §13.03(b)(3)(A) to require laboratories to report all hepatitis B virus (HBV) DNA test results, including negative results. Anticancer therapy should not be delayed for the results of these screening tests.
As per reports, it has been revealed that the accused manager has been. COBAS AmpliPrep/COBAS TaqMan HBV Test, v2.0 is an FDA-approved in vitro nucleic acid amplification test for the quantification of hepatitis B virus (HBV) DNA in human serum. The risk of hepatitis B virus (HBV) transmission by blood transfusion (estimated at 1 in 63,000-1 in 5,000 units in the United States) exceeds that of hepatitis C virus (HCV) or human immunodeficiency virus (HIV).
It aids in assessing viral response to antiviral treatment as measured by changes in plasma HBV DNA levels. LabCorp test details for Hepatitis B Virus (HBV), Quantitative, DNA Real-time PCR, (Graphical). Screen and vaccinate all household and sexual susceptible contacts.
HBV DNA Quantitative PCR Test is a simple blood test that is done to diagnose and confirm the presence of Hepatitis B Virus in the infected patients. Hepatitis B Virus DNA (HBV DNA) Testing and Interpretation Update Page 3 of 3 LAB-SD-031-003 References Roche Molecular Systems. This test determines how contagious you are.
OBI poses a risk for the development of cirrhosis and hepatocellular carcinoma. The presence of HBeAg is associated with higher HBV DNA levels, thus, increased infectiousness. Tests—hepatitis B surface antigen (HBsAg), hepatitis B core antibody (anti-H) total immunoglobulin (Ig) or IgG, and antibody to hepatitis B surface antigen (anti-HBs)—prior to, or at the beginning of, systemic anticancer therapy.
A positive HBV-DNA level (greater than 115 copies of the virus per mL > IU/mL) indicates that the virus is multiplying in the individual’s body and the person is contagious. IMPORTANT INSTRUCTIONS *Test results released pertain to the specimen submitted.*All test results are dependent on the quality of the sample received by the Laboratory. I suggest to maintain a strict follow up checking also HBeAg/anti-HBe status, ALT level, HBV DNA, quantitative.
Testing for this antigen can also be used to monitor the effectiveness of treatment for HBV. Log-rank test, P < 0. 1 IU/mL of HBV DNA is approximately 5. copies/mL A negative result (“Not Detected”) does not rule out the presence of HBV below the limit of detection of the assay or the presence of inhibitory substances.
You will get a discount of up to 46% over the market price on HBV DNA Quantitative PCR price in Delhi when booking through us. HBV stands for Hepatitis B Virus, DNA stands for Deoxyribonucleic acid, and Quantitative Polymerase Chain Reaction (PCR) is the procedure that is used to derive the value of the amount of present. Eight (11%) of the participants had HBV DNA in their sweat.
HCV RNA is a marker of viral replication and persistence is associated with disease progression. This test quantifies HCV RNA of free HCV virions in serum / plasma. The results between two methods with AlkPhos.
The hepatitis B virus test is used for various reasons. Occult hepatitis B infection (OBI) is defined as the presence of hepatitis B virus (HBV) DNA in the liver or serum in the absence of detectable HBV surface antigen (HBsAg). Labs usually measure down to less than 0 IU/mL.
It aids in assessing viral response to antiviral treatment as measured by changes in plasma HBV DNA levels. Stable at room temperature for 24 hours or refrigerated for six days. This test is intended for use as an aid in management of HBV infected patients and is not intended for use in the initial diagnosis or confirmation of HBV infection.
Test for HIV and HCV;. In occult hepatitis B, serum DNA levels are frequently below the limits of detection for many of the quantitative assays. Hepatitis b dna test in hindi | hbv dna test in hindi | hbv dna pcr qualitative test | hbv dna - Duration:.
The prevalence of OBI in Kenya is unknown, thus a study was undertaken to determine the prevalence and molecular characterization of OBI in. LabCorp test details for Hepatitis B Virus (HBV), Quantitative, DNA Real-time PCR, (Nongraphical).

Hepatitis B Virus Hbv Www 6ebweb Com
Gale Onefile Health And Medicine Document The Effect Of Modified Sini Decoction On Survival Rates Of Patients With Hepatitis B Virus Related Acute On Chronic Liver Failure

Detection Of Low Copy Number Integrated Viral Dna Formed By In Vitro Hepatitis B Infection Protocol

Anti Dsdna Antibodies Wikipedia
Hepatitis B Virus Tests Hepatitis B Virus Test Results Hepatitis B Treatment Dr Lal Pathlabs
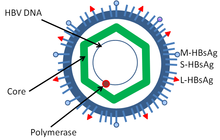
Hepatitis B Wikipedia

Guide To Hbv Dna Test Hepatitis B Virus In India Upto 40 Discount In More Than 22 Cities India S Largest Medical Test Platform
348dhwykjliljm

Hepatitis B Panel Of Blood Test Hepatitis B Blood Test Youtube

50 Discount On Blood Tests Mri Pet Scan Ct Scan India S Largest Medical Test Platform

Detection Of Low Copy Number Integrated Viral Dna Formed By In Vitro Hepatitis B Infection Protocol

All You Need To Know About Hepatitis B Infection And Hbsag Test Blog Hod

Detection Of Low Copy Number Integrated Viral Dna Formed By In Vitro Hepatitis B Infection Protocol

Addressing Hepatitis B In Africa Hepatitis B Foundation

Hepatitis B Wikipedia

Antiviral Efficacy Of Qust Saussurea Lappa And Afsanteen Artemisia Absinthium For Chronic Hepatitis B A Prospective Single Arm Pilot Clinical Trial Ansari S Siddiqui Ma Malhotra S Maaz M Phcog Res

Islamic Videos Hepatitis B Ka Ilaj Aur Alamat In Urdu And Hindi

Hbsag Blood Test In Hindi Youtube

Hepatitis B Dna Test In Hindi Hbv Dna Test In Hindi Hbv Dna Pcr Qualitative Test Hbv Dna Youtube

Antituberculosis Therapy In Patients With Hepatitis B Viral Infection Patel Nd Singh Sp Hep B Annual

Hepatitis Bs Surface Antigen Hbsag Purpose Normal Range Of Results 1mg
Gale Academic Onefile Document Using Machine Learning Algorithms To Predict Hepatitis B Surface Antigen Seroclearance
Ancient Mummy S Dna Reveals Hepatitis B Virus Existed Since 16th Centuries Buzz News India Tv

Hbeag Negative Chronic Hepatitis B An Overview Ma Fazle Akbar S M Hep B Annual
Hepatitis B Foundation Understanding Your Hepatitis B Test Results
Gale Onefile Health And Medicine Document Hepatitis B Virus Infection In Tanzania Current Status And Challenges

Experience With Hepatitis B Viral Load Testing In Jharkhand
World Hepatitis Day Significance Theme Types Of Hepatitis You Must Know About

The Biological Meaning Of Anti Hbc Positive Result In Blood Donors Relation To Hbv Dna And To Other Serological Markers

Hepatitis B Non Reactive 686 Questions Answered Practo Consult
Q Tbn 3aand9gcs63bfbubfw Kkeypovc Vzqxn9xzpx0ltsax 4e3jrns X2pv4 Usqp Cau

Hepatitis B Test Hbeab Hepatitis B Antibody Procedure Purpose Results Cost Price

Hepatitis B E Antigen Hbeag Test In Hindi Hbeag Negative Means Hbeag Positive Means Youtube

Guide To Hbv Dna Test Hepatitis B Virus In India Upto 40 Discount In More Than 22 Cities India S Largest Medical Test Platform

Systematic Reviews And Evidence Summaries Who Guidelines On Hepatitis B And C Testing Ncbi Bookshelf
Hepatitis B Virus Infectious Disease And Antimicrobial Agents
Recombinant Dna Wikipedia

Systematic Reviews And Evidence Summaries Who Guidelines On Hepatitis B And C Testing Ncbi Bookshelf

Hepatitis B Vaccine Wikipedia
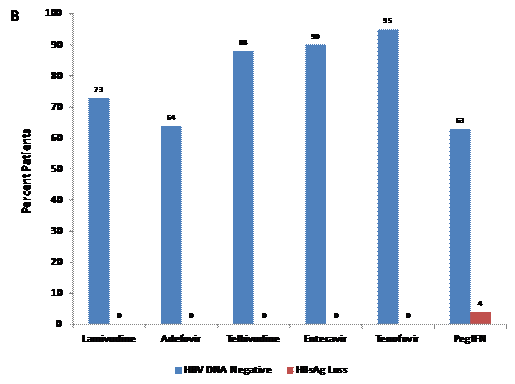
Hbsag Levels Linked With Fibrosis In Hbeag Positive Patients Hepatitis B Foundation

Guide To Hbv Dna Test Hepatitis B Virus In India Upto 40 Discount In More Than 22 Cities India S Largest Medical Test Platform
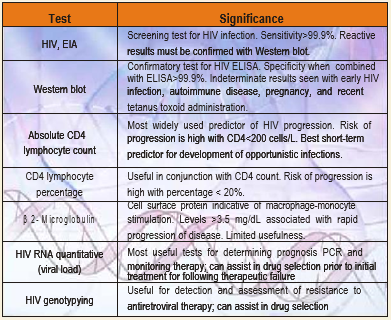
Hiv Virus Load By Real Time Pcr Rt Pcr For Diagnosis Of Hiv

Prelims Question Paper 19 In Hindi

Why Some Hbs Ag Positive Patients Are Negative For Hbv Dna

Serum Hepatitis B Surface Antigen Levels Correlate With High Serum Hbv Dna Levels In Patients With Chronic Hepatitis B A Cross Sectional Study Gupta E Kumar A Choudhary A Kumar M Sarin S K
Gale Onefile Health And Medicine Document Hepatitis B Virus Infection In Tanzania Current Status And Challenges
Hepatitis B Research Review February Hepatitis B Foundation
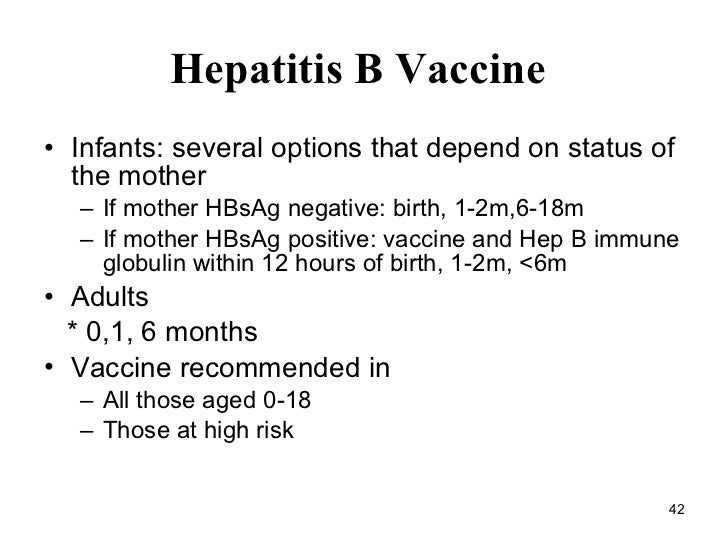
Hepatitis Ppt Final
Diagnosed With Chronic Hepatitis B What Does Your Hbv Dna Test Viral Load Tell You Hepatitis B Foundation

Pdf A Clinical Study On Hepatitis B Under The Influence Of Vasadi Kwath
Q Tbn 3aand9gctnczk Zeoncwquzv2jaw2oul3fzjozccljyt0z9wy9fcfvlsjh Usqp Cau
Nbknxpjmwhpawm

No 342 Hepatitis B And Pregnancy Coalition For Global Hepatitis Elimination
Gale Academic Onefile Document Towards Genotype Specific Care For Chronic Hepatitis B The First 6 Years Follow Up From The Charm Cohort Study
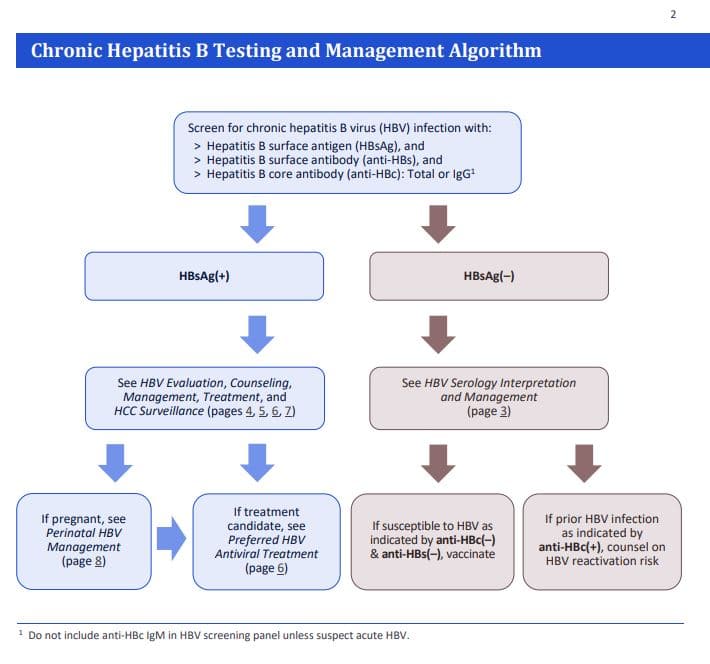
Hepatitis B Prevention Archives Hepatitis B Foundation

Hepatitis B Is Not A Genetic Disease And Here S Why Hepatitis B Foundation

Hepatitis B Lab Tests Hep
Gale Onefile Health And Medicine Document Optimizing The Use Of The Gamma Glutamyl Transpeptidase To Platelet Ratio And Transient Elastography To Identify Liver Cirrhosis In Patients With Chronic Hepatitis B Concurrent With Nonalcoholic
Www Hepatitisc Uw Edu Pdf Treatment Infection Monitoring Core Concept All

Hbv Dna Test In Hindi Hbv Dna Quantitative Test In Hindi Hbv Dna Viral Load Hbv Dna Report Youtube

Hepatitis B Dna Test In Hindi Hbv Dna Test In Hindi Hbv Dna Pcr Qualitative Test Hbv Dna Youtube

Serum Hepatitis B Surface Antigen Levels Correlate With High Serum Hbv Dna Levels In Patients With Chronic Hepatitis B A Cross Sectional Study Gupta E Kumar A Choudhary A Kumar M Sarin S K
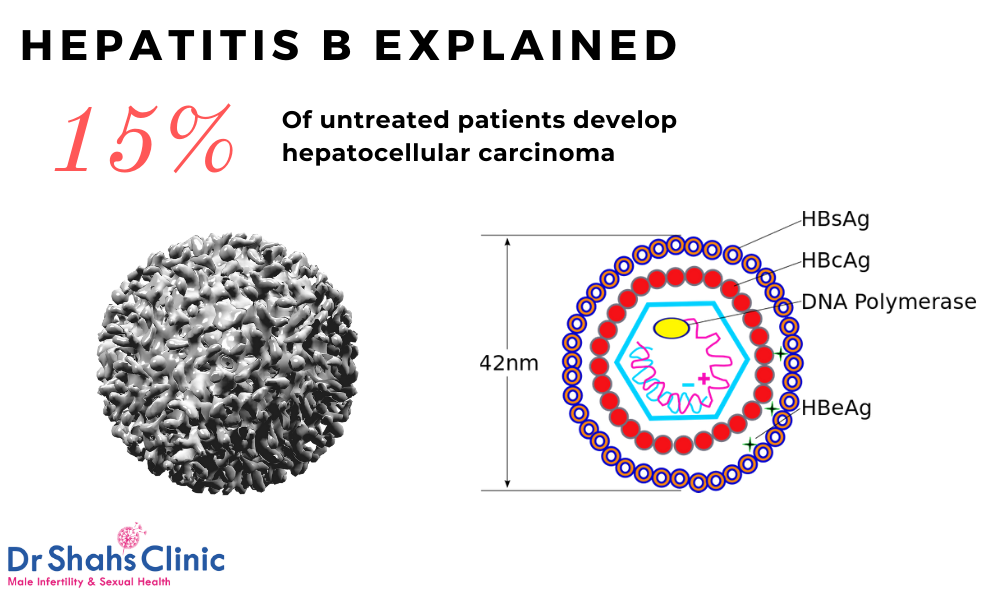
Hepatitis B Treatment In Chennai Dr Shahs Clinic

Australia Antigen Symptoms Treatment Video Lesson Transcript Study Com
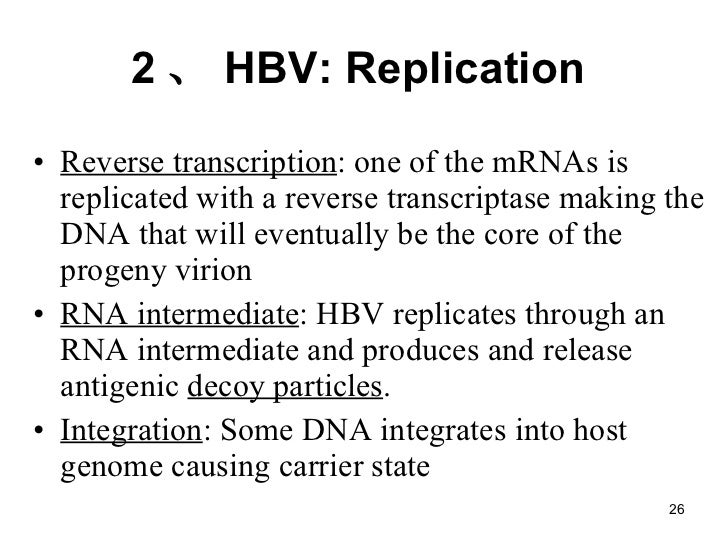
Hepatitis Ppt Final

Hbsag Test Hepatitis B Surface Antigen Test यह ट स ट क य क य ज त ह इस ट स ट स क य पत चलत ह Youtube

Pdf Prevalence Of Hepatitis B Seromarkers And Hepatitis C Antibodies In Blood Donors In Basra Iraq
Gale Onefile Health And Medicine Document Quantitative Anti Hbc In Liver Pathological States In Patients With Chronic Hepatitis B Virus Infection
Gale Onefile Health And Medicine Document Quantitative Anti Hbc In Liver Pathological States In Patients With Chronic Hepatitis B Virus Infection

Full Text Analysis Of The Seroprevalence Of Hiv Hbsag Hcv And Syphilitic Infections Detected In The Pretranfusion Blood A Short Report International Journal Of Blood Transfusion And Immunohematology Ijbti
Q Tbn 3aand9gcrfrv1do4gvyexwx0mt67l7ttwivvfsoqztaany3ndfhxi9vk7b Usqp Cau

Guide To Hbv Dna Test Hepatitis B Virus In India Upto 40 Discount In More Than 22 Cities India S Largest Medical Test Platform
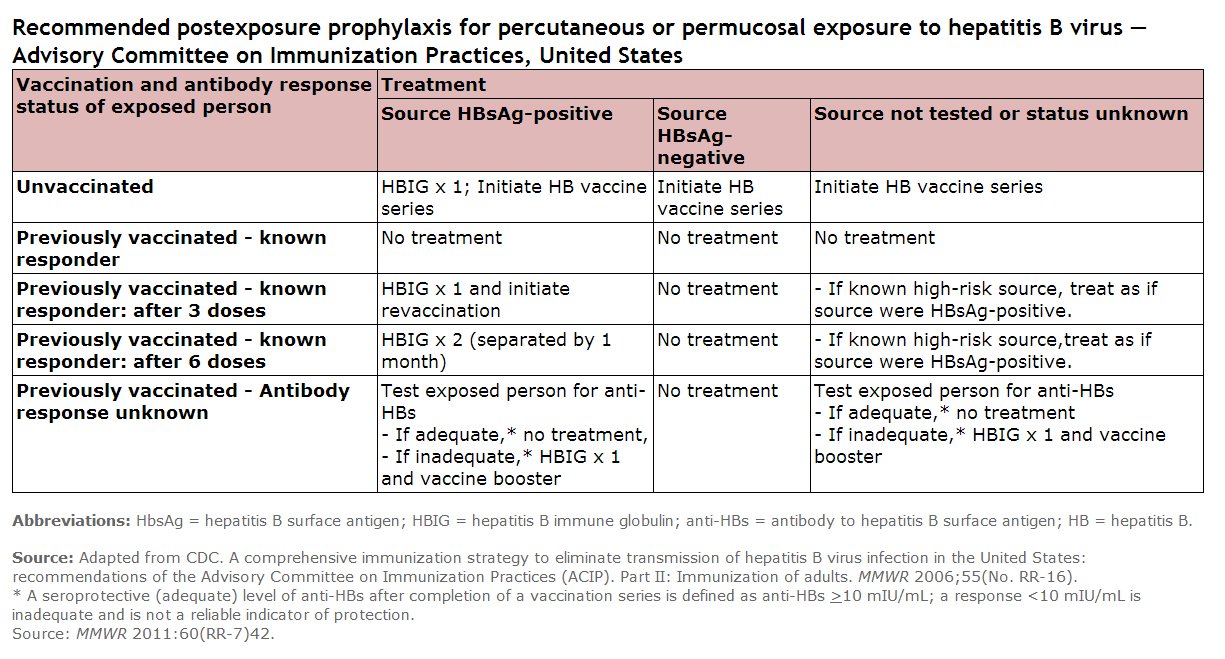
Hepatitis B In Pregnancy

Hbsag Test Procedure Purpose Results Cost Price
Gale Academic Onefile Document Hepatitis B Virus Testing And Care Among Pregnant Women Using Commercial Claims Data United States 11 14
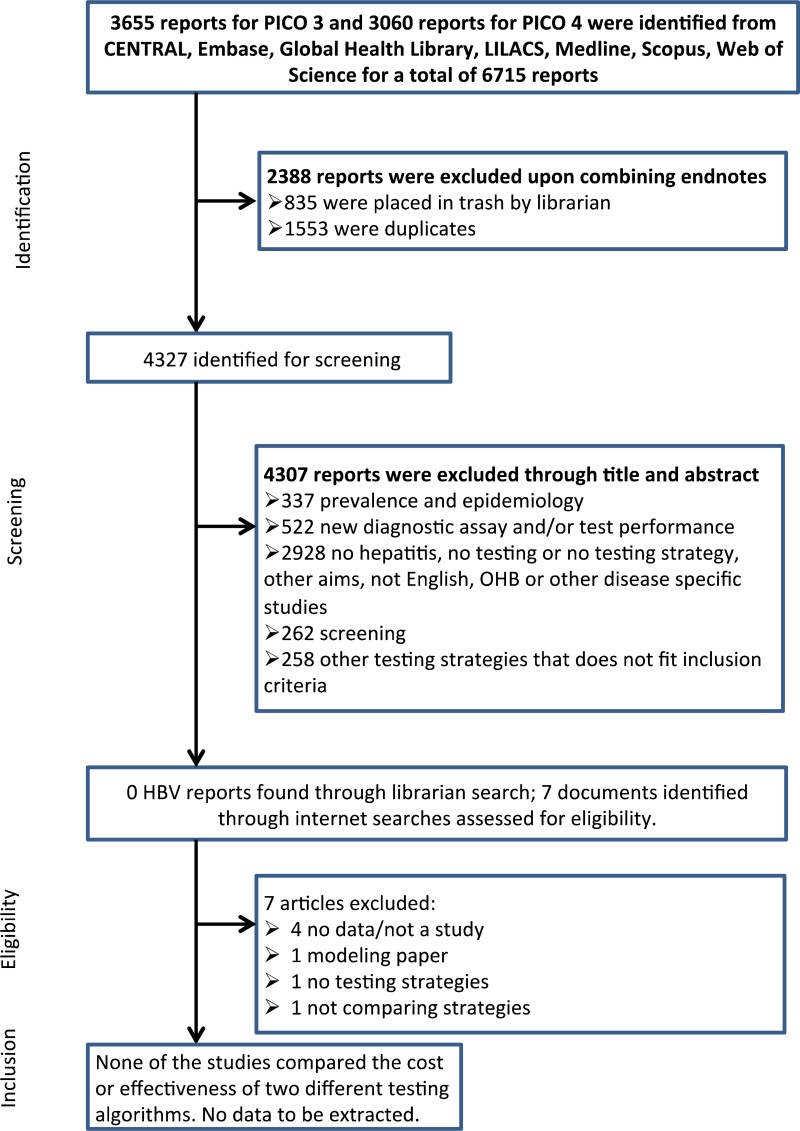
Systematic Reviews And Evidence Summaries Who Guidelines On Hepatitis B And C Testing Ncbi Bookshelf
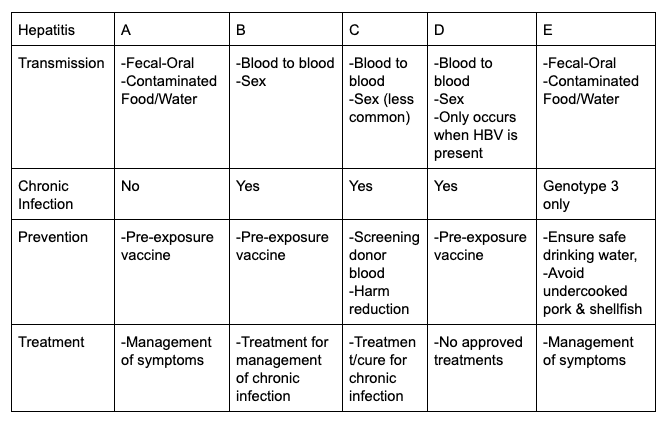
Hepatitis B Foundation Page 2 Of 48 Baruch S Blumberg Institute

Hbsag Test Hbsag Test In Hindi Hbv Test In Hindi Hbsag Rapid Test In Hindi Hbsag ट स ट ह द Youtube

Hbv Dna Test In Hindi Hbv Dna Quantitative Test In Hindi Hbv Dna Viral Load Hbv Dna Report Youtube

Hbsag Blood Test In Hindi Youtube

Lamivir Hbv Tablet View Uses Side Effects Price And Substitutes 1mg

Genomic Population Health And The Genomic Dna Test The University Of Vermont Health Network
Q Tbn 3aand9gcrsjfwq9tfbcillqab2myungi4z5num5ronisuhikreivjig7q9 Usqp Cau

Genetic Testing Wikipedia

Hiv Virus Load By Real Time Pcr Rt Pcr For Diagnosis Of Hiv
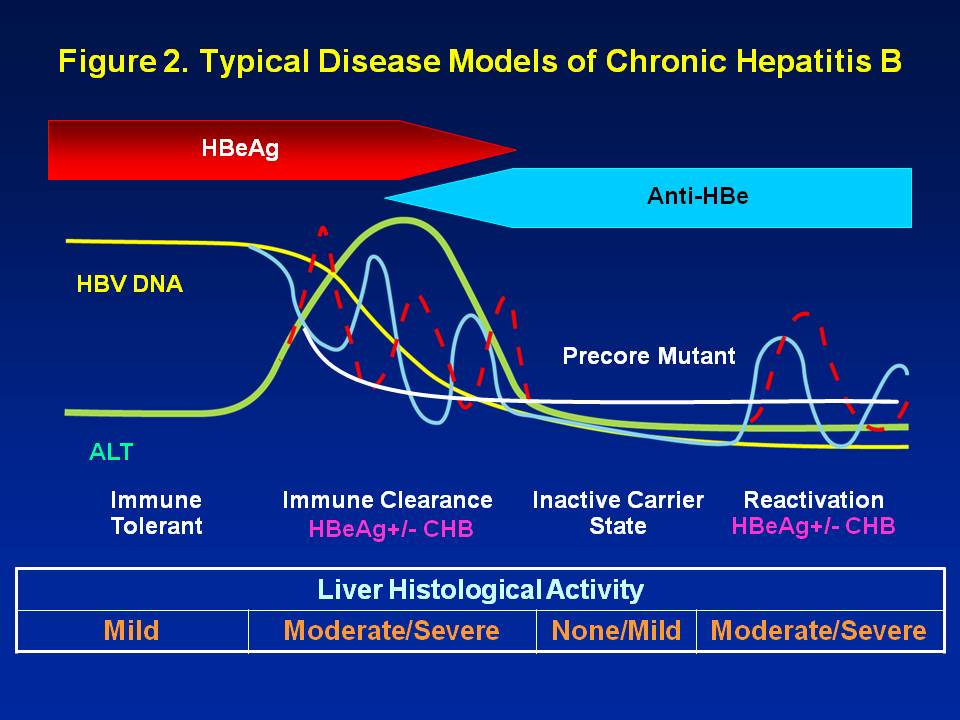
Diagnosed With Chronic Hepatitis B What Phase Hbeag Positive Chronic Hepatitis Immune Reactive Immune Clearance Hepatitis B Foundation
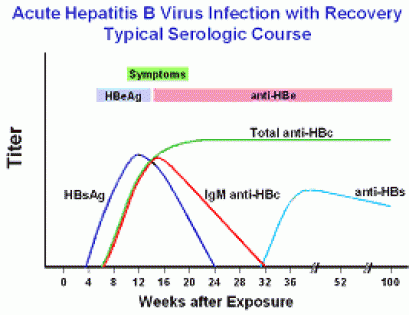
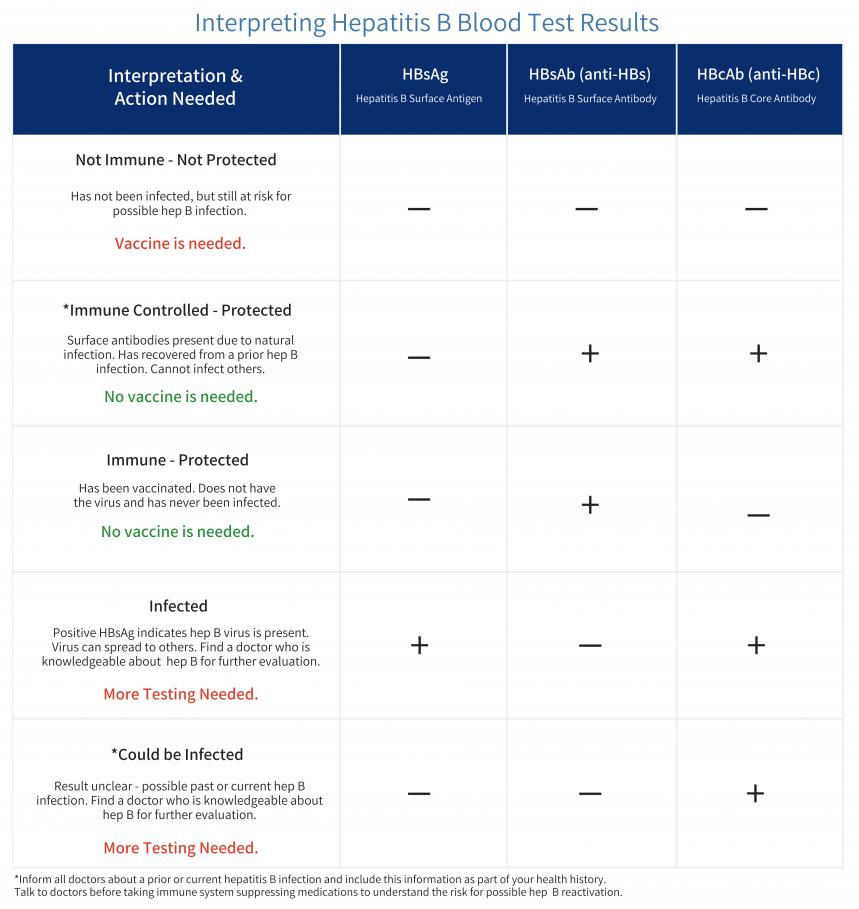
Hepatitis B Foundation Understanding Your Hepatitis B Test Results
Mutation Profiling Of The Hepatitis B Virus Strains Circulating In North Indian Population

Pdf Chapter 1 Literature Review

What Is Difference Between Hbs Ag And Hbe Ag Does Hbe Ag Negative Person Carries Hbv Virus Quora
Mutation Profiling Of The Hepatitis B Virus Strains Circulating In North Indian Population

Antituberculosis Therapy In Patients With Hepatitis B Viral Infection Patel Nd Singh Sp Hep B Annual
Gale Academic Onefile Document Prevalence And Clinical Relevance Of Occult Hepatitis B In The Fibrosis Progression And Antiviral Response To Inf Therapy In Hiv Hcv Coinfected Patients

Management Of Mother To Child Transmission Of Hepatitis B Virus Propositions And Challenges Sciencedirect

50 Off On Hbv Dna Test Cost Starting From 2450 Only
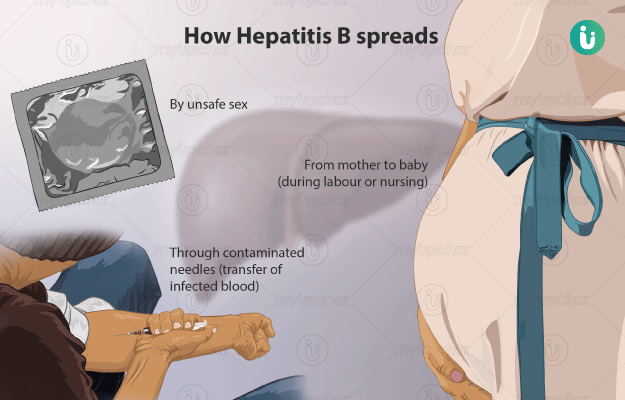
Hepatitis B Symptoms Causes Treatment Medicine Prevention Diagnosis
Www Childrensmn Org References Lab Serology Hepatitis B Dna Quantitative Pcr Pdf
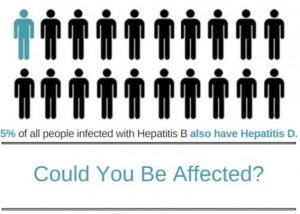
The Medical Community Wakes Up To A Dangerous Threat To People With Hepatitis B Coinfection With Hepatitis D Hepatitis B Foundation



